Abciximab
Reopro (abciximab) is an antibody pharmaceutical. Abciximab was first approved as Reopro on 1994-12-22. It is used to treat unstable angina in the USA. The pharmaceutical is active against integrin beta-3. In addition, it is known to target integrin alpha-IIb.
Download report
Favorite
Commercial
Trade Name
FDA
EMA
No data
Drug Products
FDA
EMA
Reference product - 351(a)
Reference product - 351(a)
Interchangeable product - 351(k)
Interchangeable product - 351(k)
Biosimilar product - 351(k)
Biosimilar product - 351(k)
Abciximab
Tradename | Proper name | Company | Number | Date | Products |
|---|---|---|---|---|---|
| Reopro | abciximab | Johnson & Johnson | N-103575 DISCN | 1994-12-22 | 1 products |
Hide discontinued
Labels
FDA
EMA
No data
Indications
FDA
EMA
Indication | Ontology | MeSH | ICD-10 |
|---|---|---|---|
| unstable angina | EFO_1000985 | D000789 | I20.0 |
Agency Specific
FDA
EMA
No data
Patent Expiration
No data
HCPCS
Code | Description |
|---|---|
| G9531 | Patient has documentation of ventricular shunt, brain tumor, multisystem trauma, or is currently taking an antiplatelet medication including: abciximab, anagrelide, cangrelor, cilostazol, clopidogrel, dipyridamole, eptifibatide, prasugrel, ticlopidine, ticagrelor, tirofiban, or vorapaxar |
| J0130 | Injection abciximab, 10 mg |
Clinical
Clinical Trials
47 clinical trials
View more details

Mock data
Subscribe for the real data
Subscribe for the real data
Indications Phases 4
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Myocardial infarction | D009203 | EFO_0000612 | I21 | — | 1 | 5 | 10 | 1 | 16 |
| Inferior wall myocardial infarction | D056989 | EFO_1000983 | — | 2 | 1 | 2 | — | 5 | |
| Unstable angina | D000789 | EFO_1000985 | I20.0 | — | 1 | 2 | 1 | — | 4 |
| St elevation myocardial infarction | D000072657 | — | — | 1 | 2 | 1 | 4 | ||
| Coronary artery disease | D003324 | I25.1 | — | — | — | 1 | 2 | 3 | |
| Coronary disease | D003327 | — | — | — | 3 | — | 3 | ||
| Ischemia | D007511 | EFO_0000556 | — | — | — | 3 | — | 3 | |
| Diabetes mellitus | D003920 | EFO_0000400 | E08-E13 | — | — | — | 1 | — | 1 |
| Acute coronary syndrome | D054058 | EFO_0005672 | — | — | — | 1 | — | 1 | |
| Middle cerebral artery infarction | D020244 | EFO_1001045 | G46.0 | — | — | — | 1 | — | 1 |
Show 2 more
Indications Phases 3
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Stroke | D020521 | EFO_0000712 | I63.9 | — | 2 | 3 | — | — | 5 |
| Balloon angioplasty coronary | D015906 | EFO_0003951 | — | — | 3 | — | — | 3 | |
| Pathologic constriction | D003251 | — | — | 1 | — | — | 1 | ||
| Arterial occlusive diseases | D001157 | EFO_0009085 | — | 1 | 1 | — | — | 1 | |
| Acute disease | D000208 | — | — | 1 | — | — | 1 | ||
| Brain ischemia | D002545 | I67.82 | — | — | 1 | — | — | 1 |
Indications Phases 1
No data
Indications Without Phase
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Anterior wall myocardial infarction | D056988 | EFO_1000812 | — | — | — | — | 1 | 1 | |
| Myocardial ischemia | D017202 | EFO_1001375 | I20-I25 | — | — | — | — | 1 | 1 |
| Atherosclerosis | D050197 | EFO_0003914 | I25.1 | — | — | — | — | 1 | 1 |
Epidemiology
Epidemiological information for investigational and approved indications
View more details
Drug
General
| Drug common name | ABCIXIMAB |
| INN | abciximab |
| Description | Abciximab (chimeric Fab) |
| Classification | Antibody |
| Drug class | monoclonal antibodies |
| Image (chem structure or protein) | 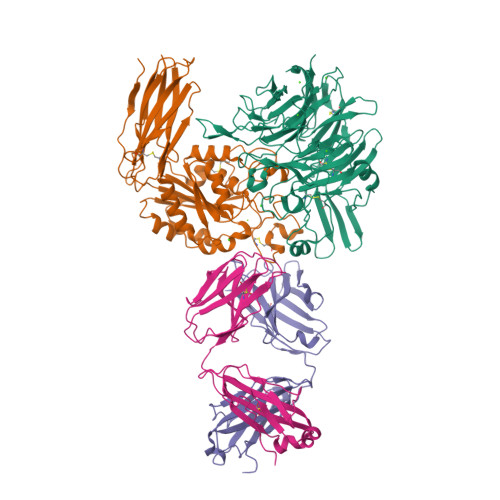 |
| Structure (InChI/SMILES or Protein Sequence) | >6V4P:C|Abciximab, heavy chain
EVQLQQSGTVLARPGASVKMSCEASGYTFTNYWMHWVKQRPGQGLEWIGAIYPGNSDTSYIQKFKGKAKLTAVTSTTSVY
MELSSLTNEDSAVYYCTLYDGYYVFAYWGQGTLVTVSAASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVSWN
SGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTH
>6V4P:D|Abciximab, light chain
EIVLTQSPVTLSVTPGDSVSLSCRASRDISNNLHWFQQTSHESPRLLIKYASQSMSGIPSRFSGSGSGTDFTLSINSVET
EDFGMYFCQQTNSWPYTFGGGTKLEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNALQSGNSQ
ESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLSSPVTKSFNRGEC |
Identifiers
| PDB | 6V4P |
| CAS-ID | 143653-53-6 |
| RxCUI | 83929 |
| ChEMBL ID | CHEMBL1201584 |
| ChEBI ID | — |
| PubChem CID | — |
| DrugBank | DB00054 |
| UNII ID | X85G7936GV (ChemIDplus, GSRS) |
Target
Agency Approved
ITGB3
ITGB3
Organism
Homo sapiens
Gene name
ITGB3
Gene synonyms
GP3A
NCBI Gene ID
Protein name
integrin beta-3
Protein synonyms
antigen CD61, CD61, GPIIIa, integrin beta 3, integrin beta chain, beta 3, integrin, beta 3 (platelet glycoprotein IIIa, antigen CD61), Platelet membrane glycoprotein IIIa
Uniprot ID
Mouse ortholog
Itgb3 (16416)
integrin beta-3 (Q3TZC6)
Alternate
ITGA2B
ITGA2B
Organism
Homo sapiens
Gene name
ITGA2B
Gene synonyms
GP2B, ITGAB
NCBI Gene ID
Protein name
integrin alpha-IIb
Protein synonyms
alphaIIb protein, CD41, GPalpha IIb, GPIIb, integrin, alpha 2b (platelet glycoprotein IIb of IIb/IIIa complex, antigen CD41), platelet fibrinogen receptor, alpha subunit, platelet glycoprotein IIb of IIb/IIIa complex, Platelet membrane glycoprotein IIb, platelet-specific antigen BAK, protein phosphatase 1, regulatory subunit 93
Uniprot ID
Mouse ortholog
Itga2b (16399)
integrin alpha-IIb (Q9Z2M0)
Variants
Clinical Variant
No data
Financial
No data
Trends
PubMed Central
Top Terms for Disease or Syndrome:

Mock data
Subscribe for the real data
Subscribe for the real data
Additional graphs summarizing 4,499 documents
View more details
Safety
Black-box Warning
No Black-box warning
Adverse Events
Top Adverse Reactions

Mock data
Subscribe for the real data
Subscribe for the real data
2,880 adverse events reported
View more details
Premium feature
Learn more about premium features at pharmakb.com
Learn more
