Humira, Trudexa(adalimumab)
Amjevita, Cyltezo, Hadlima, Hulio, Humira, Hyrimoz, Idacio, Yusimry (adalimumab) is an antibody pharmaceutical. Adalimumab was first approved as Humira on 2002-12-31. It is used to treat ankylosing spondylitis, crohn disease, hidradenitis suppurativa, juvenile arthritis, and psoriatic arthritis amongst others in the USA. It has been approved in Europe to treat ankylosing spondylitis, arthritis, crohn disease, hidradenitis suppurativa, and juvenile arthritis amongst others. The pharmaceutical is active against tumor necrosis factor.
Download report
Favorite
COVID-19
Top 200 Pharmaceuticals by Retail Sales
Commercial
Trade Name
FDA
EMA
Amjevita, Cyltezo, Hadlima, Hulio, Humira, Hyrimoz, Idacio, Yusimry (discontinued: Abrilada)
Drug Products
FDA
EMA
Reference product - 351(a)
Reference product - 351(a)
Interchangeable product - 351(k)
Interchangeable product - 351(k)
Biosimilar product - 351(k)
Biosimilar product - 351(k)
Adalimumab
Tradename | Proper name | Company | Number | Date | Products |
|---|---|---|---|---|---|
| Humira | adalimumab | AbbVie | N-125057 RX | 2002-12-31 | 9 products |
Show 1 discontinued
Indications
FDA
EMA
Indication | Ontology | MeSH | ICD-10 |
|---|---|---|---|
| ankylosing spondylitis | EFO_0003898 | D013167 | M45 |
| crohn disease | EFO_0000384 | D003424 | K50 |
| hidradenitis suppurativa | — | D017497 | L73.2 |
| juvenile arthritis | EFO_0002609 | D001171 | M08 |
| psoriatic arthritis | EFO_0003778 | D015535 | L40.5 |
| rheumatoid arthritis | EFO_0000685 | D001172 | M06.9 |
| ulcerative colitis | EFO_0000729 | D003093 | K51 |
| uveitis | EFO_1001231 | D014605 | H20.9 |
Agency Specific
FDA
EMA
Expiration | Code | ||
|---|---|---|---|
adalimumab, Cyltezo, Boehringer Ingelheim Pharmaceuticals, Inc. | |||
| Date TBD | Interchangeable excl. | ||
adalimumab, Humira, AbbVie Inc. | |||
| 2028-02-24 | Orphan excl. | ||
Patent Expiration
No data
HCPCS
Code | Description |
|---|---|
| J0135 | Injection, adalimumab, 20 mg |
Clinical
Clinical Trials
467 clinical trials
View more details

Mock data
Subscribe for the real data
Subscribe for the real data
Indications Phases 4
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Rheumatoid arthritis | D001172 | EFO_0000685 | M06.9 | 4 | 23 | 49 | 51 | 25 | 149 |
| Psoriasis | D011565 | EFO_0000676 | L40 | 3 | 10 | 34 | 14 | 12 | 69 |
| Crohn disease | D003424 | EFO_0000384 | K50 | 2 | 7 | 29 | 16 | 15 | 65 |
| Ulcerative colitis | D003093 | EFO_0000729 | K51 | 2 | 2 | 13 | 8 | 10 | 35 |
| Psoriatic arthritis | D015535 | EFO_0003778 | L40.5 | 1 | 2 | 11 | 6 | 9 | 29 |
| Ankylosing spondylitis | D013167 | EFO_0003898 | M45 | 1 | 1 | 9 | 9 | 8 | 27 |
| Uveitis | D014605 | EFO_1001231 | H20.9 | 1 | 6 | 7 | 2 | 1 | 16 |
| Inflammatory bowel diseases | D015212 | EFO_0003767 | 1 | 1 | 2 | 5 | 8 | 16 | |
| Hidradenitis suppurativa | D017497 | L73.2 | 1 | 5 | 4 | 3 | 1 | 14 | |
| Spondylarthritis | D025241 | — | — | 1 | 5 | 1 | 7 |
Show 16 more
Indications Phases 3
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Healthy volunteers/patients | — | 23 | 1 | 1 | — | — | 24 | ||
| Juvenile arthritis | D001171 | EFO_0002609 | M08 | — | — | 5 | — | 1 | 6 |
| Inflammation | D007249 | — | 1 | 1 | — | 1 | 3 | ||
| Pyoderma gangrenosum | D017511 | EFO_0006835 | L88 | — | 2 | 1 | — | — | 3 |
| Osteoarthritis | D010003 | EFO_0002506 | M15-M19 | 1 | 2 | 1 | — | — | 3 |
| Pain | D010146 | EFO_0003843 | R52 | 1 | 1 | 1 | — | 1 | 3 |
| Behcet syndrome | D001528 | EFO_0003780 | M35.2 | — | — | 2 | — | — | 2 |
| Covid-19 | D000086382 | U07.1 | — | — | 1 | — | — | 1 | |
| Pouchitis | D019449 | EFO_0003921 | K91.850 | — | — | 1 | — | — | 1 |
| Intervertebral disc displacement | D007405 | EFO_1001800 | — | — | 1 | — | — | 1 |
Show 5 more
Indications Phases 2
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Sarcoidosis | D012507 | EFO_0000690 | D80-D89 | — | 4 | — | — | — | 4 |
| Focal segmental glomerulosclerosis | D005923 | EFO_0004236 | 1 | 2 | — | — | — | 3 | |
| Autoimmune diseases | D001327 | EFO_0000540 | M30-M36 | 1 | 2 | — | — | — | 2 |
| Choroidal neovascularization | D020256 | 1 | 1 | — | — | — | 2 | ||
| Mucopolysaccharidosis vi | D009087 | 2 | 2 | — | — | — | 2 | ||
| Mucopolysaccharidosis i | D008059 | E76.0 | 2 | 2 | — | — | — | 2 | |
| Mucopolysaccharidosis ii | D016532 | E76.1 | 2 | 2 | — | — | — | 2 | |
| Diabetes mellitus | D003920 | EFO_0000400 | E08-E13 | 1 | 1 | — | — | — | 1 |
| Type 1 diabetes mellitus | D003922 | EFO_0001359 | E10 | 1 | 1 | — | — | — | 1 |
| Hypoglycemia | D007003 | HP_0001943 | E16.2 | 1 | 1 | — | — | — | 1 |
Show 16 more
Indications Phases 1
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Uveal neoplasms | D014604 | EFO_1001230 | 1 | — | — | — | — | 1 | |
| Immune system diseases | D007154 | D89.9 | 1 | — | — | — | — | 1 | |
| Therapeutic equivalency | D013810 | 1 | — | — | — | — | 1 | ||
| Anaplastic thyroid carcinoma | D065646 | 1 | — | — | — | — | 1 | ||
| Innate immunity | D007113 | 1 | — | — | — | — | 1 | ||
| Diabetic retinopathy | D003930 | EFO_0003770 | 1 | — | — | — | — | 1 |
Indications Without Phase
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Infections | D007239 | EFO_0000544 | — | — | — | — | 1 | 1 | |
| Encephalitis | D004660 | — | — | — | — | 1 | 1 | ||
| Drug monitoring | D016903 | — | — | — | — | 1 | 1 | ||
| Fatigue | D005221 | HP_0012378 | R53.83 | — | — | — | — | 1 | 1 |
| Irritable bowel syndrome | D043183 | EFO_0000555 | K58 | — | — | — | — | 1 | 1 |
| Uveomeningoencephalitic syndrome | D014607 | Orphanet_3437 | H20.82 | — | — | — | — | 1 | 1 |
Epidemiology
Epidemiological information for investigational and approved indications
View more details
Drug
General
| Drug common name | ADALIMUMAB |
| INN | adalimumab |
| Description | Adalimumab, sold under the brand name Humira among others, is a medication used to treat rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn's disease, ulcerative colitis, psoriasis, hidradenitis suppurativa, uveitis, and juvenile idiopathic arthritis. Use is generally only recommended in people who have not responded to other treatments. It is used by injection under the skin.
|
| Classification | Antibody |
| Drug class | monoclonal antibodies |
| Image (chem structure or protein) | 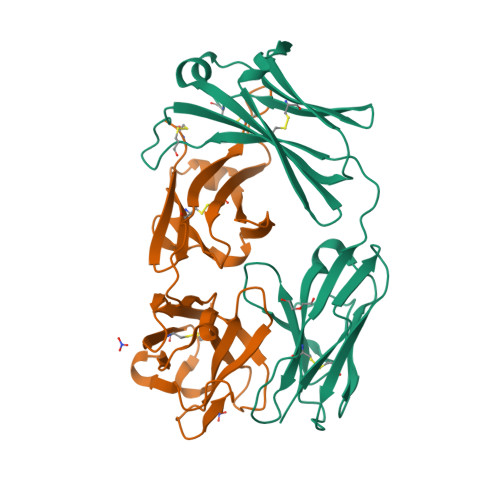 |
| Structure (InChI/SMILES or Protein Sequence) | >6CR1:H|Heavy chain of adalimumab EFab (VH-IgE CH2)
EVQLVESGGGLVQPGRSLRLSCAASGFTFDDYAMHWVRQAPGKGLEWVSAITWNSGHIDYADSVEGRFTISRDNAKNSLY
LQMNSLRAEDTAVYYCAKVSYLSTASSLDYWGQGTLVTVSSASTKGPTVKILQSICDGGGHFPPTIQLLCLVSGYTPGTI
QITWLEDGQVMDVDLSTASTTQEGELASTQSELTLSQKHWLSDRTYTCQVTYQGHTFEDSTKKCAHHHHHH
>6CR1:L|Light chain of adalimumab EFab (VL-IgE CH2)
DIQMTQSPSSLSASVGDRVTITCRASQGIRNYLAWYQQKPGKAPKLLIYAASTLQSGVPSRFSGSGSGTDFTLTISSLQP
EDVATYYCQRYNRAPYTFGQGTKVEIKRTVAAPTVKILQSSCDGGGHFPPTIQLLCLVSGYTPGTIQITWLEDGQVMDVD
LSTASTTQEGELASTQSELTLSQKHWLSDRTYTCQVTYQGHTFEDSGKKCA |
Identifiers
| PDB | 3WD5, 4NYL, 6CR1 |
| CAS-ID | 331731-18-1 |
| RxCUI | 327361 |
| ChEMBL ID | CHEMBL1201580 |
| ChEBI ID | — |
| PubChem CID | — |
| DrugBank | DB00051 |
| UNII ID | FYS6T7F842 (ChemIDplus, GSRS) |
Target
Agency Approved
TNF
TNF
Organism
Homo sapiens
Gene name
TNF
Gene synonyms
TNFA, TNFSF2
NCBI Gene ID
Protein name
tumor necrosis factor
Protein synonyms
APC1 protein, Cachectin, TNF, macrophage-derived, TNF, monocyte-derived, TNF-a, TNF-alpha, tumor necrosis factor ligand 1F, Tumor necrosis factor ligand superfamily member 2, tumor necrosis factor-alpha, tumor necrotic factor alpha
Uniprot ID
Mouse ortholog
Tnf (21926)
tumor necrosis factor (P06804)
Alternate
No data
Variants
Clinical Variant
No data
Financial
Humira - AbbVie
$
€
£
₣

Mock data
Subscribe for the real data
Subscribe for the real data

Mock data
Subscribe for the real data
Subscribe for the real data
Imraldi - Biogen
$
€
£
₣

Mock data
Subscribe for the real data
Subscribe for the real data

Mock data
Subscribe for the real data
Subscribe for the real data
Hadlima - Organon
$
€
£
₣

Mock data
Subscribe for the real data
Subscribe for the real data

Mock data
Subscribe for the real data
Subscribe for the real data
Tabular view
Trends
PubMed Central
Top Terms for Disease or Syndrome:

Mock data
Subscribe for the real data
Subscribe for the real data
Additional graphs summarizing 25,205 documents
View more details
Safety
Black-box Warning
Black-box warning for: Amjevita, Humira, Yuflyma
Adverse Events
Top Adverse Reactions

Mock data
Subscribe for the real data
Subscribe for the real data
4,107 adverse events reported
View more details
Premium feature
Learn more about premium features at pharmakb.com
Learn more
