Elspar, Spectrila(asparaginase)
Elspar, Spectrila (asparaginase) is an enzyme pharmaceutical. Asparaginase was first approved as Elspar on 1978-01-10. It is used to treat cholelithiasis, lymphoma, and precursor cell lymphoblastic leukemia-lymphoma in the USA. It has been approved in Europe to treat precursor cell lymphoblastic leukemia-lymphoma.
Download report
Favorite
Commercial
Trade Name
FDA
EMA
No data
Drug Products
FDA
EMA
Reference product - 351(a)
Reference product - 351(a)
Interchangeable product - 351(k)
Interchangeable product - 351(k)
Biosimilar product - 351(k)
Biosimilar product - 351(k)
Asparaginase
Tradename | Proper name | Company | Number | Date | Products |
|---|---|---|---|---|---|
| Elspar | asparaginase | Recordati Rare Diseases, Inc. | N-101063 DISCN | 1978-01-10 | 1 products |
Hide discontinued
Labels
FDA
EMA
No data
Agency Specific
FDA
EMA
No data
Patent Expiration
No data
Clinical
Clinical Trials
175 clinical trials
View more details

Mock data
Subscribe for the real data
Subscribe for the real data
Indications Phases 3
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Leukemia | D007938 | C95 | 2 | 20 | 21 | — | 5 | 47 | |
| Lymphoma | D008223 | C85.9 | — | 6 | 7 | — | — | 13 | |
| Myeloid leukemia acute | D015470 | C92.0 | 1 | 4 | 6 | — | — | 11 | |
| Myelodysplastic syndromes | D009190 | D46 | 1 | 1 | 5 | — | — | 7 | |
| Non-hodgkin lymphoma | D008228 | C85.9 | 1 | 3 | 1 | — | — | 5 | |
| Down syndrome | D004314 | EFO_0001064 | Q90 | — | 1 | 2 | — | — | 3 |
| Megakaryoblastic leukemia acute | D007947 | C94.2 | — | — | 2 | — | — | 2 | |
| Monocytic leukemia acute | D007948 | — | — | 2 | — | — | 2 | ||
| Myelomonocytic leukemia acute | D015479 | C92.5 | — | — | 2 | — | — | 2 | |
| Erythroblastic leukemia acute | D004915 | EFO_1001257 | C94.0 | — | — | 2 | — | — | 2 |
Show 9 more
Indications Phases 2
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| T-cell lymphoma | D016399 | 2 | 1 | — | — | 1 | 4 | ||
| Biphenotypic leukemia acute | D015456 | C95.0 | 1 | 4 | — | — | — | 4 | |
| Extranodal nk-t-cell lymphoma | D054391 | C86.0 | — | 1 | — | — | 1 | 2 | |
| Philadelphia chromosome | D010677 | 2 | 1 | — | — | — | 2 | ||
| Precursor t-cell lymphoblastic leukemia-lymphoma | D054218 | 1 | 1 | — | — | — | 2 | ||
| Hematologic neoplasms | D019337 | 1 | 1 | — | — | — | 2 | ||
| Inborn genetic diseases | D030342 | EFO_0000508 | — | 1 | — | — | — | 1 | |
| T-cell leukemia | D015458 | — | 1 | — | — | — | 1 | ||
| B-cell leukemia | D015448 | — | 1 | — | — | — | 1 | ||
| Neutropenia | D009503 | D70 | — | 1 | — | — | — | 1 |
Show 3 more
Indications Phases 1
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Hypersensitivity | D006967 | EFO_0003785 | T78.40 | 1 | — | — | — | — | 1 |
Indications Without Phase
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Ataxia telangiectasia | D001260 | Orphanet_100 | G11.3 | — | — | — | — | 1 | 1 |
Epidemiology
Epidemiological information for investigational and approved indications
View more details
Drug
General
| Drug common name | ASPARAGINASE |
| INN | crisantaspase |
| Description | Asparaginase is an enzyme that is used as a medication and in food manufacturing. As a medication, L-asparaginase is used to treat acute lymphoblastic leukemia (ALL) and lymphoblastic lymphoma (LBL). It is given by injection into a vein, muscle, or under the skin. A pegylated version is also available. In food manufacturing it is used to decrease acrylamide.
|
| Classification | Enzyme |
| Drug class | enzymes |
| Image (chem structure or protein) | 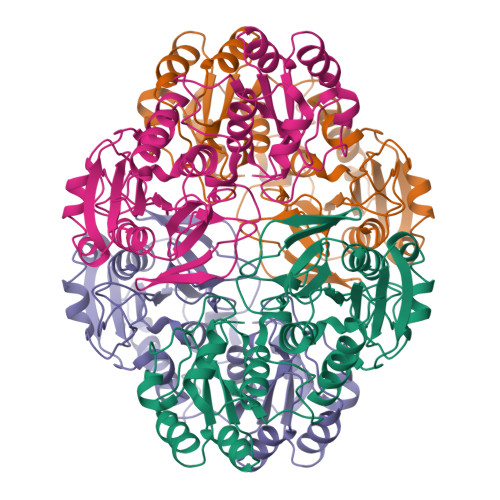 |
| Structure (InChI/SMILES or Protein Sequence) | — |
Identifiers
Target
Agency Approved
No data
Alternate
No data
Variants
Clinical Variant
No data
Financial
No data
Trends
PubMed Central
Top Terms for Disease or Syndrome:

Mock data
Subscribe for the real data
Subscribe for the real data
Additional graphs summarizing 10,351 documents
View more details
Safety
Black-box Warning
No Black-box warning
Adverse Events
Top Adverse Reactions

Mock data
Subscribe for the real data
Subscribe for the real data
177 adverse events reported
View more details
Premium feature
Learn more about premium features at pharmakb.com
Learn more
