Imfinzi(durvalumab)
Imfinzi (durvalumab) is an antibody pharmaceutical. Durvalumab was first approved as Imfinzi on 2017-05-01. It is used to treat transitional cell carcinoma in the USA. It has been approved in Europe to treat non-small-cell lung carcinoma. The pharmaceutical is active against programmed cell death 1 ligand 1.
Download report
Favorite
Top 200 Pharmaceuticals by Retail Sales
Commercial
Trade Name
FDA
EMA
Imfinzi
Drug Products
FDA
EMA
Reference product - 351(a)
Reference product - 351(a)
Interchangeable product - 351(k)
Interchangeable product - 351(k)
Biosimilar product - 351(k)
Biosimilar product - 351(k)
Durvalumab
Tradename | Proper name | Company | Number | Date | Products |
|---|---|---|---|---|---|
| Imfinzi | durvalumab | AstraZeneca UK Ltd | N-761069 RX | 2017-05-01 | 2 products |
Labels
FDA
EMA
Brand Name | Status | Last Update |
|---|---|---|
| imfinzi | Biologic Licensing Application | 2021-02-19 |
Indications
FDA
EMA
Indication | Ontology | MeSH | ICD-10 |
|---|---|---|---|
| transitional cell carcinoma | — | D002295 | — |
Agency Specific
FDA
EMA
Expiration | Code | ||
|---|---|---|---|
durvalumab, Imfinzi, AstraZeneca UK Ltd | |||
| 2029-10-21 | Orphan excl. | ||
Patent Expiration
No data
HCPCS
Code | Description |
|---|---|
| J9173 | Injection, durvalumab, 10 mg |
Clinical
Clinical Trials
620 clinical trials
View more details

Mock data
Subscribe for the real data
Subscribe for the real data
Indications Phases 4
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Non-small-cell lung carcinoma | D002289 | 33 | 90 | 31 | 1 | 3 | 146 | ||
| Neoplasms | D009369 | C80 | 38 | 26 | — | 1 | — | 52 | |
| Neoplasm metastasis | D009362 | EFO_0009708 | 1 | — | — | 1 | — | 2 |
Indications Phases 3
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Lung neoplasms | D008175 | C34.90 | 12 | 34 | 3 | — | 2 | 44 | |
| Breast neoplasms | D001943 | EFO_0003869 | C50 | 22 | 29 | 1 | — | 1 | 42 |
| Squamous cell carcinoma of head and neck | D000077195 | 17 | 20 | 3 | — | — | 33 | ||
| Liver neoplasms | D008113 | EFO_1001513 | C22.0 | 8 | 20 | 5 | — | 2 | 31 |
| Small cell lung carcinoma | D055752 | 6 | 16 | 4 | — | 3 | 28 | ||
| Ovarian neoplasms | D010051 | EFO_0003893 | C56 | 15 | 18 | 1 | — | — | 26 |
| Urinary bladder neoplasms | D001749 | C67 | 9 | 18 | 2 | — | — | 25 | |
| Renal cell carcinoma | D002292 | 7 | 12 | 2 | — | — | 18 | ||
| Biliary tract neoplasms | D001661 | C24.9 | 3 | 11 | 1 | — | — | 13 | |
| Urologic neoplasms | D014571 | C64-C68 | 3 | 8 | 2 | — | — | 11 |
Show 11 more
Indications Phases 2
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Colorectal neoplasms | D015179 | 17 | 26 | — | — | — | 34 | ||
| Pancreatic neoplasms | D010190 | EFO_0003860 | C25 | 17 | 23 | — | — | — | 32 |
| Triple negative breast neoplasms | D064726 | 10 | 13 | — | — | — | 20 | ||
| Head and neck neoplasms | D006258 | 11 | 11 | — | — | — | 14 | ||
| Melanoma | D008545 | 9 | 6 | — | — | 1 | 13 | ||
| Stomach neoplasms | D013274 | EFO_0003897 | C16 | 6 | 11 | — | — | — | 13 |
| Cholangiocarcinoma | D018281 | C22.1 | 2 | 11 | — | — | — | 13 | |
| Prostatic neoplasms | D011471 | C61 | 4 | 9 | — | — | — | 11 | |
| Uterine cervical neoplasms | D002583 | 7 | 7 | — | — | — | 11 | ||
| Esophageal neoplasms | D004938 | C15 | 4 | 9 | — | — | — | 10 |
Show 71 more
Indications Phases 1
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Primary myelofibrosis | D055728 | D47.4 | 2 | — | — | — | — | 2 | |
| Precursor cell lymphoblastic leukemia-lymphoma | D054198 | C91.0 | 1 | — | — | — | — | 1 | |
| Bcr-abl positive chronic myelogenous leukemia | D015464 | EFO_0000340 | 1 | — | — | — | — | 1 | |
| Myelomonocytic leukemia chronic | D015477 | C93.1 | 1 | — | — | — | — | 1 | |
| Anemia | D000740 | EFO_0004272 | D64.9 | 1 | — | — | — | — | 1 |
| Hodgkin disease | D006689 | C81 | 1 | — | — | — | — | 1 | |
| Leukemia myeloid chronic atypical bcr-abl negative | D054438 | C92.2 | 1 | — | — | — | — | 1 | |
| Myeloid leukemia chronic-phase | D015466 | 1 | — | — | — | — | 1 | ||
| Thrombocytosis | D013922 | D75.83 | 1 | — | — | — | — | 1 | |
| Polycythemia | D011086 | EFO_0005804 | D75.1 | 1 | — | — | — | — | 1 |
Show 12 more
Indications Without Phase
No data
Epidemiology
Epidemiological information for investigational and approved indications
View more details
Drug
General
| Drug common name | DURVALUMAB |
| INN | durvalumab |
| Description | Durvalumab (human mab) |
| Classification | Antibody |
| Drug class | monoclonal antibodies |
| Image (chem structure or protein) | 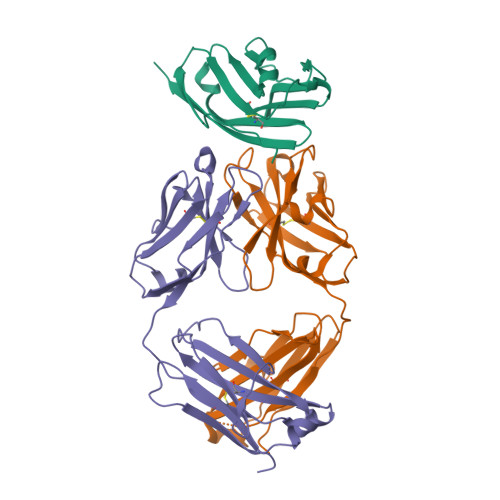 |
| Structure (InChI/SMILES or Protein Sequence) | >5X8M:B|durvalumab heavy chain
EVQLVESGGGLVQPGGSLRLSCAASGFTFSRYWMSWVRQAPGKGLEWVANIKQDGSEKYYVDSVKGRFTISRDNAKNSLY
LQMNSLRAEDTAVYYCAREGGWFGELAFDYWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTV
SWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTHHHHHH
>5X8M:C|durvalumab light chain
EIVLTQSPGTLSLSPGERATLSCRASQRVSSSYLAWYQQKPGQAPRLLIYDASSRATGIPDRFSGSGSGTDFTLTISRLE
PEDFAVYYCQQYGSLPWTFGQGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNALQSGNS
QESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLSSPVTKSFNRGEC |
Identifiers
| PDB | 5X8M, 5XJ4 |
| CAS-ID | 1428935-60-7 |
| RxCUI | 1919503 |
| ChEMBL ID | CHEMBL3301587 |
| ChEBI ID | — |
| PubChem CID | — |
| DrugBank | DB11714 |
| UNII ID | 28X28X9OKV (ChemIDplus, GSRS) |
Target
Agency Approved
CD274
CD274
Alternate
No data
Variants
Clinical Variant
No data
Financial
Imfinzi - AstraZeneca
$
€
£
₣

Mock data
Subscribe for the real data
Subscribe for the real data

Mock data
Subscribe for the real data
Subscribe for the real data
Tabular view
Trends
PubMed Central
Top Terms for Disease or Syndrome:

Mock data
Subscribe for the real data
Subscribe for the real data
Additional graphs summarizing 13,280 documents
View more details
Safety
Black-box Warning
No Black-box warning
Adverse Events
Top Adverse Reactions

Mock data
Subscribe for the real data
Subscribe for the real data
71,483 adverse events reported
View more details
Premium feature
Learn more about premium features at pharmakb.com
Learn more
