Raptiva(efalizumab)
Raptiva (efalizumab) is an antibody pharmaceutical. Efalizumab was first approved as Raptiva on 2003-10-27. It is used to treat psoriasis in the USA. It has been approved in Europe to treat psoriasis. The pharmaceutical is active against integrin alpha-L.
Download report
Favorite
Commercial
Therapeutic Areas
Therapeutic Area | MeSH |
|---|---|
| skin and connective tissue diseases | D017437 |
Trade Name
FDA
EMA
No data
Drug Products
FDA
EMA
Reference product - 351(a)
Reference product - 351(a)
Interchangeable product - 351(k)
Interchangeable product - 351(k)
Biosimilar product - 351(k)
Biosimilar product - 351(k)
Efalizumab
Tradename | Proper name | Company | Number | Date | Products |
|---|---|---|---|---|---|
| Raptiva | efalizumab | Genentech | N-125075 DISCN | 2003-10-27 | 1 products |
Hide discontinued
Labels
FDA
EMA
No data
Indications
FDA
EMA
Indication | Ontology | MeSH | ICD-10 |
|---|---|---|---|
| psoriasis | EFO_0000676 | D011565 | L40 |
Agency Specific
FDA
EMA
No data
Patent Expiration
No data
HCPCS
No data
Clinical
Clinical Trials
41 clinical trials
View more details

Mock data
Subscribe for the real data
Subscribe for the real data
Indications Phases 4
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Psoriasis | D011565 | EFO_0000676 | L40 | 2 | 1 | 7 | 11 | 1 | 21 |
Indications Phases 3
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Kidney transplantation | D016030 | 1 | 2 | 1 | — | — | 2 |
Indications Phases 2
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Type 1 diabetes mellitus | D003922 | EFO_0001359 | E10 | 2 | 4 | — | — | — | 4 |
| Atopic dermatitis | D003876 | EFO_0000274 | L20 | 1 | 1 | — | — | — | 2 |
| Autoimmune diseases | D001327 | EFO_0000540 | M30-M36 | 1 | 1 | — | — | — | 1 |
| Chronic kidney failure | D007676 | EFO_0003884 | N18.6 | 1 | 1 | — | — | — | 1 |
| Hypoglycemia | D007003 | HP_0001943 | E16.2 | 1 | 1 | — | — | — | 1 |
| Sjogren's syndrome | D012859 | EFO_0000699 | M35.0 | — | 1 | — | — | — | 1 |
| Rheumatoid arthritis | D001172 | EFO_0000685 | M06.9 | — | 1 | — | — | — | 1 |
Indications Phases 1
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Macular edema | D008269 | 2 | — | — | — | — | 2 | ||
| Macular degeneration | D008268 | EFO_0001365 | H35.30 | 1 | — | — | — | — | 1 |
| Uveitis | D014605 | EFO_1001231 | H20.9 | 1 | — | — | — | — | 1 |
| Hidradenitis suppurativa | D017497 | L73.2 | 1 | — | — | — | — | 1 | |
| Psoriatic arthritis | D015535 | EFO_0003778 | L40.5 | 1 | — | — | — | — | 1 |
Epidemiology
Epidemiological information for investigational and approved indications
View more details
Drug
General
| Drug common name | EFALIZUMAB |
| INN | efalizumab |
| Description | Immunoglobulin G1, anti-(human antigen CD11a) (human-mouse monoclonal hu1124 gamma1-chain), disulfide with human-mouse monoclonal hu1124 light chain, dimer |
| Classification | Antibody |
| Drug class | Fc fusion protein; monoclonal antibodies |
| Image (chem structure or protein) | 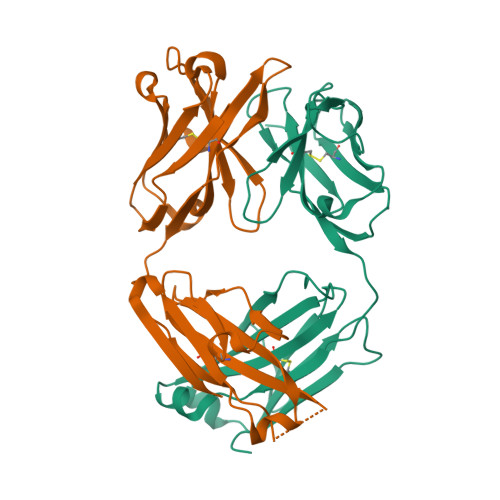 |
| Structure (InChI/SMILES or Protein Sequence) | >3EO9:H|Efalizumab Fab fragment, heavy chain
EVQLVESGGGLVQPGGSLRLSCAASGYSFTGHWMNWVRQAPGKGLEWVGMIHPSDSETRYNQKFKDRFTISVDKSKNTLY
LQMNSLRAEDTAVYYCARGIYFYGTTYFDYWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTV
SWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKV
>3EO9:L|Efalizumab Fab fragment, light chain
DIQMTQSPSSLSASVGDRVTITCRASKTISKYLAWYQQKPGKAPKLLIYSGSTLQSGVPSRFSGSGSGTDFTLTISSLQP
EDFATYYCQQHNEYPLTFGQGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNALQSGNSQ
ESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLSSPVTKSFNRGEC |
Identifiers
| PDB | 3EO9, 3EOA, 3EOB |
| CAS-ID | 214745-43-4 |
| RxCUI | 356988 |
| ChEMBL ID | CHEMBL1201575 |
| ChEBI ID | — |
| PubChem CID | — |
| DrugBank | DB00095 |
| UNII ID | XX2MN88N5D (ChemIDplus, GSRS) |
Target
Agency Approved
ITGAL
ITGAL
Organism
Homo sapiens
Gene name
ITGAL
Gene synonyms
CD11A
NCBI Gene ID
Protein name
integrin alpha-L
Protein synonyms
antigen CD11A (p180), lymphocyte function-associated antigen 1, alpha polypeptide, CD11 antigen-like family member A, CD11a, integrin gene promoter, integrin, alpha L (antigen CD11A (p180), lymphocyte function-associated antigen 1; alpha polypeptide), Leukocyte adhesion glycoprotein LFA-1 alpha chain, Leukocyte function-associated molecule 1 alpha chain, LFA-1 alpha, LFA-1A, lymphocyte function-associated antigen 1, alpha polypeptide
Uniprot ID
Mouse ortholog
Itgal (16408)
integrin alpha-L (Q3U159)
Alternate
No data
Variants
Clinical Variant
No data
Financial
No data
Trends
PubMed Central
Top Terms for Disease or Syndrome:

Mock data
Subscribe for the real data
Subscribe for the real data
Additional graphs summarizing 1,685 documents
View more details
Safety
Black-box Warning
No Black-box warning
Adverse Events
Top Adverse Reactions

Mock data
Subscribe for the real data
Subscribe for the real data
3,414 adverse events reported
View more details
Premium feature
Learn more about premium features at pharmakb.com
Learn more
