Follitropin
Gonal-f, Pergoveris (follitropin) is a protein pharmaceutical. Follitropin was first approved as Gonal-f on 1995-10-20. It has been approved in Europe to treat anovulation, assisted reproductive techniques, female infertility, and hypogonadism. The pharmaceutical is active against follicle-stimulating hormone receptor.
Download report
Favorite
Commercial
Therapeutic Areas
Therapeutic Area | MeSH |
|---|---|
| urogenital diseases | D000091642 |
| endocrine system diseases | D004700 |
| therapeutics | D013812 |
| investigative techniques | D008919 |
| reproductive and urinary physiological phenomena | D012101 |
Trade Name
FDA
EMA
No data
Drug Products
FDA
EMA
New Drug Application (NDA)
New Drug Application (NDA)
Abbreviated New Drug Application (ANDA)
Abbreviated New Drug Application (ANDA)
No data
Labels
FDA
EMA
Brand Name | Status | Last Update |
|---|---|---|
| gonal-f rff | Biologic Licensing Application | 2022-08-08 |
Indications
FDA
EMA
No data
Agency Specific
FDA
EMA
No data
Patent Expiration
No data
Clinical
Clinical Trials
177 clinical trials
View more details

Mock data
Subscribe for the real data
Subscribe for the real data
Indications Phases 4
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Infertility | D007246 | EFO_0000545 | 5 | 9 | 25 | 21 | 22 | 80 | |
| Female infertility | D007247 | EFO_0008560 | N97 | 4 | — | 9 | 4 | 1 | 18 |
| Ovulation induction | D010062 | — | 4 | 5 | 2 | 1 | 12 | ||
| Fertilization in vitro | D005307 | — | 1 | 2 | 4 | 3 | 10 | ||
| Polycystic ovary syndrome | D011085 | EFO_0000660 | E28.2 | 1 | 1 | 3 | 2 | 2 | 9 |
| Anovulation | D000858 | — | — | 4 | 1 | 1 | 6 | ||
| Healthy volunteers/patients | — | 4 | — | — | 1 | — | 5 | ||
| Hypogonadism | D007006 | E23.0 | — | 1 | 1 | 1 | 1 | 4 | |
| Assisted reproductive techniques | D027724 | — | — | 2 | 1 | 1 | 4 | ||
| Aneuploidy | D000782 | — | — | — | 1 | 1 | 2 |
Show 4 more
Indications Phases 3
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Pregnancy | D011247 | EFO_0002950 | Z33.1 | — | — | 1 | — | 4 | 5 |
| Fertility | D005298 | — | — | 1 | — | 1 | 2 | ||
| Oocyte donation | D018587 | — | — | 1 | — | 1 | 2 | ||
| Embryo transfer | D004624 | — | — | 1 | — | — | 1 | ||
| Male infertility | D007248 | EFO_0004248 | N46 | — | 1 | 1 | — | — | 1 |
Indications Phases 2
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Fertilization | D005306 | — | 1 | — | — | — | 1 |
Indications Without Phase
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Primary ovarian insufficiency | D016649 | EFO_0004266 | E28.3 | — | — | — | — | 3 | 3 |
| Pregnancy rate | D018873 | — | — | — | — | 1 | 1 | ||
| Prostatic neoplasms | D011471 | C61 | — | — | — | — | 1 | 1 | |
| Thalassemia | D013789 | EFO_1001996 | D56 | — | — | — | — | 1 | 1 |
| Reproduction | D012098 | — | — | — | — | 1 | 1 | ||
| Pregnancy outcome | D011256 | — | — | — | — | 1 | 1 | ||
| Endometriosis | D004715 | EFO_0001065 | N80 | — | — | — | — | 1 | 1 |
| Galactosemias | D005693 | E74.21 | — | — | — | — | 1 | 1 | |
| Female genital diseases | D005831 | EFO_0009549 | N85 | — | — | — | — | 1 | 1 |
| Oxidative stress | D018384 | EFO_1001905 | — | — | — | — | 1 | 1 |
Show 1 more
Epidemiology
Epidemiological information for investigational and approved indications
View more details
Drug
General
| Drug common name | FOLLITROPIN |
| INN | follitropin alfa |
| Description | Gonadotropin preparations are drugs that mimic the physiological effects of gonadotropins, used therapeutically mainly as fertility medication for ovarian hyperstimulation and ovulation induction. For example, the so-called menotropins consist of LH and FSH extracted from human urine from menopausal women. There are also recombinant variants.
|
| Classification | Protein |
| Drug class | tricyclic compounds; atropine derivatives |
| Image (chem structure or protein) | 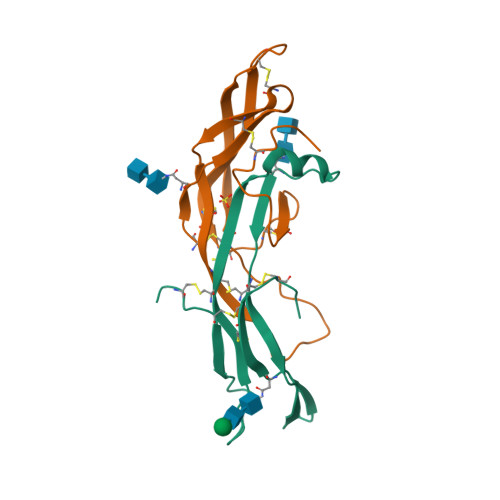 |
| Structure (InChI/SMILES or Protein Sequence) | — |
Target
Variants
Clinical Variant
No data
Financial
No data
Trends
PubMed Central
Top Terms for Disease or Syndrome:

Mock data
Subscribe for the real data
Subscribe for the real data
Additional graphs summarizing 1,657 documents
View more details
Safety
Black-box Warning
No Black-box warning
Adverse Events
Top Adverse Reactions

Mock data
Subscribe for the real data
Subscribe for the real data
1,663 adverse events reported
View more details
Premium feature
Learn more about premium features at pharmakb.com
Learn more
