Insulin
Insuman (insulin) is a protein pharmaceutical. Insulin was first approved as Insuman on 1997-02-21. It has been approved in Europe to treat diabetes mellitus. The pharmaceutical is active against insulin receptor, insulin-like growth factor II, and insulin-like growth factor I. In addition, it is known to target relaxin-3 receptor 1, relaxin receptor 2, transient receptor potential cation channel subfamily V member 2, relaxin receptor 1, relaxin-3 receptor 2, and insulin-like growth factor 1 receptor.
Download report
Favorite
Top 200 Pharmaceuticals by Retail Sales
Commercial
Trade Name
FDA
EMA
No data
Drug Products
FDA
EMA
New Drug Application (NDA)
New Drug Application (NDA)
Abbreviated New Drug Application (ANDA)
Abbreviated New Drug Application (ANDA)
No data
Labels
FDA
EMA
Brand Name | Status | Last Update |
|---|---|---|
| esika extreme moisturizing spf 16 | OTC monograph final | 2013-12-20 |
| lantus solostar | Biologic Licensing Application | 2023-06-06 |
Indications
FDA
EMA
No data
Agency Specific
FDA
EMA
No data
Patent Expiration
No data
ATC Codes
A: Alimentary tract and metabolism drugs
— A10: Drugs used in diabetes
— A10A: Insulins and analogues
— A10AB: Insulins and analogues for injection, fast-acting
— A10AB01: Insulin (human)
— A10AB02: Insulin (beef)
— A10AB03: Insulin (pork)
— A10AB04: Insulin lispro
— A10AB05: Insulin aspart
— A10AB06: Insulin glulisine
— A10AC: Insulins and analogues for injection, intermediate-acting
— A10AC01: Insulin (human)
— A10AC02: Insulin (beef)
— A10AC03: Insulin (pork)
— A10AC04: Insulin lispro
— A10AD: Insulins and analogues for injection used in diabetes, intermediate- or long-acting combined with fast-acting
— A10AD01: Insulin (human)
— A10AD02: Insulin (beef)
— A10AD03: Insulin (pork)
— A10AD04: Insulin lispro
— A10AD05: Insulin aspart
— A10AD06: Insulin degludec and insulin aspart
— A10AE: Insulins and analogues for injection, long-acting
— A10AE01: Insulin (human)
— A10AE02: Insulin (beef)
— A10AE03: Insulin (pork)
— A10AE04: Insulin glargine
— A10AE05: Insulin detemir
— A10AE06: Insulin degludec
— A10AE54: Insulin glargine and lixisenatide
— A10AE56: Insulin degludec and liraglutide
— A10AF: Insulins and analogues for inhalation
— A10AF01: Insulin (human)
HCPCS
Code | Description |
|---|---|
| A4221 | Supplies for maintenance of non-insulin drug infusion catheter, per week (list drugs separately) |
| A4224 | Supplies for maintenance of insulin infusion catheter, per week |
| A4225 | Supplies for external insulin infusion pump, syringe type cartridge, sterile, each |
| A4226 | Supplies for maintenance of insulin infusion pump with dosage rate adjustment using therapeutic continuous glucose sensing, per week |
| A4230 | Infusion set for external insulin pump, non needle cannula type |
| A4231 | Infusion set for external insulin pump, needle type |
| A4232 | Syringe with needle for external insulin pump, sterile, 3 cc |
| A9274 | External ambulatory insulin delivery system, disposable, each, includes all supplies and accessories |
| E0784 | External ambulatory infusion pump, insulin |
| E0787 | External ambulatory infusion pump, insulin, dosage rate adjustment using therapeutic continuous glucose sensing |
Show 21 more
Clinical
Clinical Trials
2167 clinical trials
View more details

Mock data
Subscribe for the real data
Subscribe for the real data
Indications Phases 4
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Type 2 diabetes mellitus | D003924 | EFO_0001360 | E11 | 71 | 54 | 262 | 260 | 235 | 874 |
| Type 1 diabetes mellitus | D003922 | EFO_0001359 | E10 | 152 | 88 | 124 | 90 | 243 | 675 |
| Diabetes mellitus | D003920 | EFO_0000400 | E08-E13 | 23 | 21 | 35 | 29 | 45 | 143 |
| Healthy volunteers/patients | — | 87 | 2 | 1 | 2 | 1 | 93 | ||
| Hyperglycemia | D006943 | HP_0003074 | R73.9 | 6 | 8 | 12 | 29 | 28 | 80 |
| Insulin resistance | D007333 | EFO_0002614 | 7 | 3 | 1 | 1 | 24 | 35 | |
| Gestational diabetes | D016640 | HP_0009800 | O24.4 | — | 6 | 11 | 6 | 14 | 34 |
| Hypoglycemia | D007003 | HP_0001943 | E16.2 | 3 | 1 | 1 | 5 | 15 | 24 |
| Critical illness | D016638 | — | 3 | 2 | 1 | 5 | 10 | ||
| Heart failure | D006333 | EFO_0003144 | I50 | 1 | 4 | 1 | 2 | 2 | 9 |
Show 49 more
Indications Phases 3
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Obesity | D009765 | EFO_0001073 | E66.9 | 6 | — | 2 | — | 19 | 27 |
| Alzheimer disease | D000544 | EFO_0000249 | F03 | 2 | 9 | 1 | — | 1 | 11 |
| Cystic fibrosis | D003550 | EFO_0000390 | E84 | — | 2 | 7 | — | 3 | 10 |
| Pregnancy | D011247 | EFO_0002950 | Z33.1 | — | 2 | 3 | — | 4 | 8 |
| Chronic obstructive pulmonary disease | D029424 | EFO_0000341 | J44.9 | 1 | 1 | 4 | — | 2 | 8 |
| Asthma | D001249 | EFO_0000270 | J45 | 1 | — | 5 | — | — | 6 |
| Glucose intolerance | D018149 | HP_0000833 | R73.03 | 1 | — | 1 | — | 3 | 5 |
| Diabetes complications | D048909 | — | 3 | 4 | — | 1 | 5 | ||
| Sepsis | D018805 | A41.9 | — | 2 | 2 | — | 2 | 5 | |
| Hypogonadism | D007006 | E23.0 | 2 | 1 | 1 | — | 1 | 4 |
Show 26 more
Indications Phases 2
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Cognitive dysfunction | D060825 | G31.84 | 1 | 9 | — | — | 3 | 13 | |
| Hyperinsulinism | D006946 | HP_0000842 | E16.1 | 3 | 1 | — | — | 4 | 8 |
| Delirium | D003693 | R41.0 | 1 | 2 | — | — | 2 | 4 | |
| Exercise | D015444 | EFO_0000483 | — | 1 | — | — | 2 | 3 | |
| Parkinson disease | D010300 | EFO_0002508 | G20 | — | 3 | — | — | — | 3 |
| Inflammation | D007249 | — | 1 | — | — | 1 | 2 | ||
| Pain | D010146 | EFO_0003843 | R52 | — | 1 | — | — | 1 | 2 |
| Premature birth | D047928 | EFO_0003917 | O60 | — | 2 | — | — | — | 2 |
| Multiple system atrophy | D019578 | — | 1 | — | — | 1 | 2 | ||
| Wounds and injuries | D014947 | T14.8 | 2 | 2 | — | — | — | 2 |
Show 18 more
Indications Phases 1
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Sarcopenia | D055948 | EFO_1000653 | M62.84 | 1 | — | — | — | 3 | 4 |
| Prediabetic state | D011236 | EFO_1001121 | R73.03 | 1 | — | — | — | 2 | 3 |
| Carpal tunnel syndrome | D002349 | EFO_0004143 | G56.0 | 1 | — | — | — | 1 | 2 |
| Glaucoma | D005901 | EFO_0000516 | H40 | 2 | — | — | — | — | 2 |
| Myocardial ischemia | D017202 | EFO_1001375 | I20-I25 | 1 | — | — | — | 1 | 2 |
| Heart valve diseases | D006349 | EFO_0009551 | I08 | 1 | — | — | — | 1 | 2 |
| Intracranial aneurysm | D002532 | EFO_0003870 | I67.1 | 1 | — | — | — | 1 | 2 |
| Neoplasms | D009369 | C80 | 1 | — | — | — | 1 | 2 | |
| Down syndrome | D004314 | EFO_0001064 | Q90 | 1 | — | — | — | — | 1 |
| Aging | D000375 | GO_0007568 | R41.81 | 1 | — | — | — | — | 1 |
Show 10 more
Indications Without Phase
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Liver diseases | D008107 | EFO_0001421 | K70-K77 | — | — | — | — | 2 | 2 |
| Infections | D007239 | EFO_0000544 | — | — | — | — | 2 | 2 | |
| Fetal macrosomia | D005320 | — | — | — | — | 2 | 2 | ||
| Orthostatic hypotension | D007024 | I95.1 | — | — | — | — | 2 | 2 | |
| Infertility | D007246 | EFO_0000545 | — | — | — | — | 2 | 2 | |
| Chronic hepatitis c | D019698 | EFO_0004220 | B18.2 | — | — | — | — | 2 | 2 |
| Pediatric obesity | D063766 | — | — | — | — | 2 | 2 | ||
| Therapeutic equivalency | D013810 | — | — | — | — | 2 | 2 | ||
| Artificial pancreas | D019397 | — | — | — | — | 2 | 2 | ||
| Spinal cord injuries | D013119 | EFO_1001919 | — | — | — | — | 2 | 2 |
Show 67 more
Epidemiology
Epidemiological information for investigational and approved indications
View more details
Drug
General
| Drug common name | INSULIN |
| INN | insulin icodec |
| Description | Recombinant human insulin |
| Classification | Protein |
| Drug class | — |
| Image (chem structure or protein) | 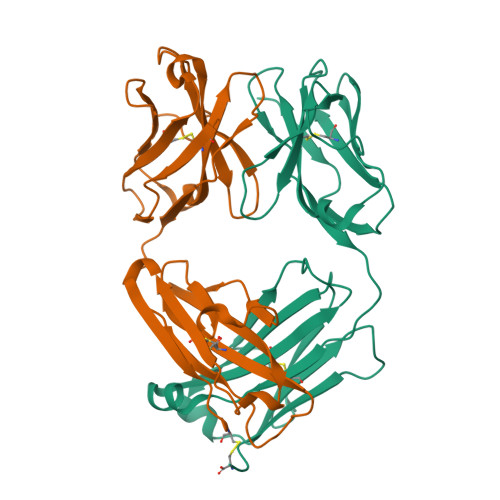 |
| Structure (InChI/SMILES or Protein Sequence) | >6Z7X:A|OXI-005 Fab Light chain
DIQMTQSPSSLSASLGGRVTITCKASQDINKYLAWYQHKPGKGPRLLIHYTSTLQPGIPSRFSGSGSGRDYSFSISNLEP
EDVATYYCLQYDSLLSFGAGTKLELKRADAAPTVSIFPPSSEQLTSGGASVVCFLNNFYPKDINVKWKIDGSERQNGVLN
SWTDQDSKDSTYSMSSTLTLTKDEYERHNSYTCEATHKTSTSPIVKSFNRNEC
>6Z7X:B|OXI-005 Fab Heavy chain
EVQLVESGGGLVKPGGSLKLSCTASGFAFSDYDMSWVRQTPEKRLEWVAFISNGGYSTYYPDTVKGRFTISRDNAENTLY
LQMSSLKSEDTAIYYCARQGLRYFDYWGLGTTLTVSSAKTTPPSVYPLAPGSAAQTNSMVTLGCLVKGYFPEPVTVTWNS
GSLSSGVHTFPAVLQSDLYTLSSSVTVPSSTWPSETVTCNVAHPASSTKVDKKIVPRDCG |
Identifiers
Target
Alternate
RXFP3
RXFP3
RXFP2
RXFP2
TRPV2
TRPV2
RXFP1
RXFP1
RXFP4
RXFP4
IGF1R
IGF1R
Organism
Homo sapiens
Gene name
RXFP3
Gene synonyms
GPCR135, RLN3R1, SALPR
NCBI Gene ID
Protein name
relaxin-3 receptor 1
Protein synonyms
G protein-coupled receptor SALPR, G-protein coupled receptor GPCR135, G-protein coupled receptor SALPR, Relaxin family peptide receptor 3, relaxin/insulin like family peptide receptor 3, RLN3 receptor 1, somatostatin and angiotensin-like peptide receptor, Somatostatin- and angiotensin-like peptide receptor
Uniprot ID
Mouse ortholog
Rxfp3 (239336)
relaxin-3 receptor 1 (Q8BGE9)
Variants
Clinical Variant
No data
Financial
Insulin - Amphastar Pharmaceuticals
$
€
£
₣

Mock data
Subscribe for the real data
Subscribe for the real data

Mock data
Subscribe for the real data
Subscribe for the real data
Insulin - Novo Nordisk
$
€
£
₣

Mock data
Subscribe for the real data
Subscribe for the real data

Mock data
Subscribe for the real data
Subscribe for the real data
Tabular view
Trends
PubMed Central
Top Terms for Disease or Syndrome:

Mock data
Subscribe for the real data
Subscribe for the real data
Additional graphs summarizing 749,311 documents
View more details
Safety
Black-box Warning
No Black-box warning
Adverse Events
Top Adverse Reactions

Mock data
Subscribe for the real data
Subscribe for the real data
2,023 adverse events reported
View more details
Premium feature
Learn more about premium features at pharmakb.com
Learn more
