Interferon beta
Avonex, Rebif (interferon beta) is a protein pharmaceutical. Interferon beta was first approved as Avonex on 1997-03-13. It has been approved in Europe to treat multiple sclerosis. The pharmaceutical is active against interleukin-6 receptor subunit alpha and interleukin-6. In addition, it is known to target interferon alpha/beta receptor 1.
Download report
Favorite
COVID-19
Commercial
Trade Name
FDA
EMA
No data
Drug Products
FDA
EMA
New Drug Application (NDA)
New Drug Application (NDA)
Abbreviated New Drug Application (ANDA)
Abbreviated New Drug Application (ANDA)
No data
Labels
FDA
EMA
Brand Name | Status | Last Update |
|---|---|---|
| avonex avonex pen | Biologic Licensing Application | 2020-12-16 |
| rebif rebif rebidose | Biologic Licensing Application | 2020-10-28 |
Indications
FDA
EMA
No data
Agency Specific
FDA
EMA
No data
Patent Expiration
No data
HCPCS
Code | Description |
|---|---|
| J1826 | Injection, interferon beta-1a, 30 mcg |
| J1830 | Injection, interferon beta-1b, 0.25 mg (code may be used for medicare when drug administered under the direct supervision of a physician, not for use when drug is self administered) |
| Q3027 | Injection, interferon beta-1a, 1 mcg for intramuscular use |
| Q3028 | Injection, interferon beta-1a, 1 mcg for subcutaneous use |
Clinical
Clinical Trials
248 clinical trials
View more details

Mock data
Subscribe for the real data
Subscribe for the real data
Indications Phases 4
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Multiple sclerosis | D009103 | EFO_0003885 | G35 | 6 | 18 | 22 | 14 | 46 | 104 |
| Relapsing-remitting multiple sclerosis | D020529 | EFO_0003929 | 4 | 12 | 23 | 23 | 21 | 82 | |
| Covid-19 | D000086382 | U07.1 | — | 8 | 2 | 2 | 2 | 14 | |
| Optic neuritis | D009902 | EFO_0007405 | H46 | — | — | — | 2 | — | 2 |
| Transverse myelitis | D009188 | G37.3 | — | — | — | 1 | — | 1 | |
| Neuritis | D009443 | — | — | — | 1 | — | 1 | ||
| Demyelinating diseases | D003711 | — | — | — | 1 | — | 1 | ||
| Myelitis | D009187 | G04.91 | — | — | — | 1 | — | 1 |
Indications Phases 3
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Respiratory distress syndrome | D012128 | EFO_1000637 | J80 | 1 | 1 | 2 | — | — | 3 |
| Hiv infections | D015658 | EFO_0000764 | B20 | 1 | — | 1 | — | 1 | 3 |
| Pneumonia | D011014 | EFO_0003106 | J18 | — | — | 1 | — | — | 1 |
| Severe acute respiratory syndrome-related coronavirus | D045473 | NCBITaxon_227859 | — | 1 | 1 | — | — | 1 | |
| Coronavirus | D017934 | — | — | 1 | — | — | 1 | ||
| Middle east respiratory syndrome coronavirus | D065207 | — | 1 | 1 | — | — | 1 | ||
| Hepatitis c | D006526 | B19.2 | — | — | 1 | — | — | 1 | |
| Chronic hepatitis c | D019698 | EFO_0004220 | B18.2 | — | — | 1 | — | — | 1 |
| Macular edema | D008269 | — | — | 1 | — | — | 1 | ||
| Intermediate uveitis | D015867 | EFO_1000986 | — | — | 1 | — | — | 1 |
Indications Phases 2
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Merkel cell carcinoma | D015266 | EFO_1001471 | C4A | 2 | 2 | — | — | — | 2 |
| Melanoma | D008545 | 1 | 1 | — | — | — | 2 | ||
| Asthma | D001249 | EFO_0000270 | J45 | — | 2 | — | — | — | 2 |
| Ulcerative colitis | D003093 | EFO_0000729 | K51 | — | 2 | — | — | — | 2 |
| Polyomavirus infections | D027601 | EFO_0007451 | 1 | 1 | — | — | — | 1 | |
| Neoplasms | D009369 | C80 | 1 | 1 | — | — | — | 1 | |
| Non-small-cell lung carcinoma | D002289 | 1 | 1 | — | — | — | 1 | ||
| Neuroendocrine carcinoma | D018278 | 1 | 1 | — | — | — | 1 | ||
| Chronic obstructive pulmonary disease | D029424 | EFO_0000341 | J44.9 | — | 1 | — | — | — | 1 |
| Multiple organ failure | D009102 | EFO_1001373 | — | 1 | — | — | — | 1 |
Show 10 more
Indications Phases 1
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Healthy volunteers/patients | — | 9 | — | — | — | — | 9 | ||
| Chronic progressive multiple sclerosis | D020528 | 1 | — | — | — | 2 | 3 | ||
| Pharmacokinetics | D010599 | 1 | — | — | — | — | 1 | ||
| Uveal neoplasms | D014604 | EFO_1001230 | 1 | — | — | — | — | 1 | |
| Therapeutic equivalency | D013810 | 1 | — | — | — | — | 1 | ||
| Endometrial neoplasms | D016889 | EFO_0004230 | 1 | — | — | — | — | 1 | |
| Endometrioid carcinoma | D018269 | 1 | — | — | — | — | 1 | ||
| Acinar cell carcinoma | D018267 | 1 | — | — | — | — | 1 | ||
| Myelodysplastic syndromes | D009190 | D46 | 1 | — | — | — | — | 1 | |
| Myeloid leukemia acute | D015470 | C92.0 | 1 | — | — | — | — | 1 |
Show 12 more
Indications Without Phase
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Metabolic bone diseases | D001851 | HP_0000938 | — | — | — | — | 1 | 1 | |
| Prenatal exposure delayed effects | D011297 | — | — | — | — | 1 | 1 | ||
| Pregnancy | D011247 | EFO_0002950 | Z33.1 | — | — | — | — | 1 | 1 |
| Adrenoleukodystrophy | D000326 | Orphanet_43 | E71.52 | — | — | — | — | 1 | 1 |
| Cytomegalovirus retinitis | D017726 | EFO_1001302 | — | — | — | — | 1 | 1 |
Epidemiology
Epidemiological information for investigational and approved indications
View more details
Drug
General
| Drug common name | INTERFERON BETA |
| INN | interferon beta |
| Description | The type-I interferons (IFN) are cytokines which play essential roles in inflammation, immunoregulation, tumor cells recognition, and T-cell responses. In the human genome, a cluster of thirteen functional IFN genes is located at the 9p21.3 cytoband over approximately 400 kb including coding genes for IFNα (IFNA1, IFNA2, IFNA4, IFNA5, IFNA6, IFNA7, IFNA8, IFNA10, IFNA13, IFNA14, IFNA16, IFNA17 and IFNA21), IFNω (IFNW1), IFNɛ (IFNE), IFNк (IFNK) and IFNβ (IFNB1), plus 11 IFN pseudogenes.
|
| Classification | Protein |
| Drug class | — |
| Image (chem structure or protein) | 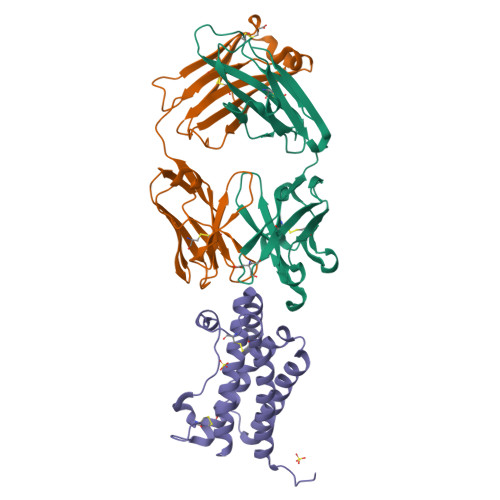 |
| Structure (InChI/SMILES or Protein Sequence) | >4CNI:A,H|OLOKIZUMAB HEAVY CHAIN, FAB PORTION
EVQLVESGGGLVQPGGSLRLSCAASGFNFNDYFMNWVRQAPGKGLEWVAQMRNKNYQYGTYYAESLEGRFTISRDDSKNS
LYLQMNSLKTEDTAVYYCARESYYGFTSYWGQGTLVTVSSASTKGPSVFPLAPCSRSTSESTAALGCLVKDYFPEPVTVS
WNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTKTYTCNVDHKPSNTKVDKRVES
>4CNI:B,L|OLOKIZUMAB LIGHT CHAIN, FAB PORTION
DIQMTQSPSSLSASVGDRVTITCQASQDIGISLSWYQQKPGKAPKLLIYNANNLADGVPSRFSGSGSGTDFTLTISSLQP
EDFATYYCLQHNSAPYTFGQGTKLEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNALQSGNSQ
ESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLSSPVTKSFNRGEC |
Target
Agency Approved
IL6R
IL6R
IL6
IL6
Alternate
IFNAR1
IFNAR1
Organism
Homo sapiens
Gene name
IFNAR1
Gene synonyms
IFNAR
NCBI Gene ID
Protein name
interferon alpha/beta receptor 1
Protein synonyms
alpha-type antiviral protein, beta-type antiviral protein, CRF2-1, Cytokine receptor class-II member 1, Cytokine receptor family 2 member 1, IFN-alpha/beta receptor 1, IFN-R-1, IFNalpha/beta receptor 1, interferon (alpha, beta and omega) receptor 1, interferon receptor 1, interferon-alpha/beta receptor alpha chain, interferon-beta receptor 1, Type I interferon receptor 1
Uniprot ID
Mouse ortholog
Ifnar1 (15975)
interferon alpha/beta receptor 1 (Q80UJ3)
Variants
Clinical Variant
No data
Financial
Avonex - Biogen
$
€
£
₣

Mock data
Subscribe for the real data
Subscribe for the real data

Mock data
Subscribe for the real data
Subscribe for the real data
Tabular view
Trends
PubMed Central
Top Terms for Disease or Syndrome:

Mock data
Subscribe for the real data
Subscribe for the real data
Additional graphs summarizing 38,433 documents
View more details
Safety
Black-box Warning
No Black-box warning
Adverse Events
Top Adverse Reactions

Mock data
Subscribe for the real data
Subscribe for the real data
14,591 adverse events reported
View more details
Premium feature
Learn more about premium features at pharmakb.com
Learn more
