Tysabri(natalizumab)
Tysabri (natalizumab) is an antibody pharmaceutical. Natalizumab was first approved as Tysabri on 2004-11-23. It is used to treat crohn disease and multiple sclerosis in the USA. It has been approved in Europe to treat multiple sclerosis. The pharmaceutical is active against integrin alpha-4.
Download report
Favorite
Top 200 Pharmaceuticals by Retail Sales
Commercial
Trade Name
FDA
EMA
Tysabri
Drug Products
FDA
EMA
Reference product - 351(a)
Reference product - 351(a)
Interchangeable product - 351(k)
Interchangeable product - 351(k)
Biosimilar product - 351(k)
Biosimilar product - 351(k)
Natalizumab
Tradename | Proper name | Company | Number | Date | Products |
|---|---|---|---|---|---|
| Tysabri | natalizumab | Biogen | N-125104 RX | 2004-11-23 | 1 products |
Labels
FDA
EMA
Brand Name | Status | Last Update |
|---|---|---|
| tysabri | Biologic Licensing Application | 2020-06-30 |
Indications
FDA
EMA
Indication | Ontology | MeSH | ICD-10 |
|---|---|---|---|
| crohn disease | EFO_0000384 | D003424 | K50 |
| multiple sclerosis | EFO_0003885 | D009103 | G35 |
Agency Specific
FDA
EMA
No data
Patent Expiration
No data
HCPCS
Code | Description |
|---|---|
| J2323 | Injection, natalizumab, 1 mg |
Clinical
Clinical Trials
87 clinical trials
View more details

Mock data
Subscribe for the real data
Subscribe for the real data
Indications Phases 4
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Relapsing-remitting multiple sclerosis | D020529 | EFO_0003929 | 2 | 5 | 7 | 10 | 12 | 36 | |
| Multiple sclerosis | D009103 | EFO_0003885 | G35 | 1 | 4 | 2 | 9 | 14 | 29 |
| Crohn disease | D003424 | EFO_0000384 | K50 | — | 2 | 3 | 2 | 1 | 8 |
Indications Phases 3
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Chronic progressive multiple sclerosis | D020528 | 1 | 1 | 1 | — | — | 3 |
Indications Phases 2
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Rheumatoid arthritis | D001172 | EFO_0000685 | M06.9 | — | 2 | — | — | — | 2 |
| Ischemic stroke | D000083242 | — | 2 | — | — | — | 2 | ||
| Epilepsy | D004827 | EFO_0000474 | G40.9 | — | 1 | — | — | — | 1 |
| Graft vs host disease | D006086 | D89.81 | — | 1 | — | — | — | 1 | |
| Multiple myeloma | D009101 | C90.0 | 1 | 1 | — | — | — | 1 | |
| Demyelinating diseases | D003711 | — | 1 | — | — | — | 1 |
Indications Phases 1
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Schizophrenia | D012559 | EFO_0000692 | F20 | 1 | — | — | — | — | 1 |
| Inclusion body myositis | D018979 | EFO_0007323 | G72.41 | 1 | — | — | — | — | 1 |
| Sickle cell anemia | D000755 | EFO_0000697 | D57 | 1 | — | — | — | — | 1 |
Indications Without Phase
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Progressive multifocal leukoencephalopathy | D007968 | EFO_0007455 | A81.2 | — | — | — | — | 1 | 1 |
| Fatigue | D005221 | HP_0012378 | R53.83 | — | — | — | — | 1 | 1 |
Epidemiology
Epidemiological information for investigational and approved indications
View more details
Drug
General
| Drug common name | NATALIZUMAB |
| INN | natalizumab |
| Description | Immunoglobulin G 4 (human-mouse monoclonal AN1002264-chain anti-human integrin 4), disulfide with human-mouse monoclonal AN100226 light chain, dimer |
| Classification | Antibody |
| Drug class | monoclonal antibodies |
| Image (chem structure or protein) | 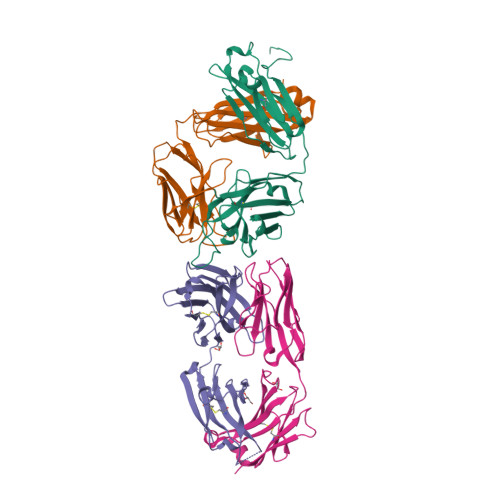 |
| Structure (InChI/SMILES or Protein Sequence) | >6FG1:A|HEAVY CHAIN OF FAB NAA32
QVHLVQSGAEVKKPGASVKVSCKASGYTFTSYTMHWVRQAPGQRLEWMGWINAGHGTTKYSQKFQGRVTITRDTSASTAY
MELSSLRSEDTAVYYCARPTSEGVAGPSRYYWYFDLWGRGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYF
PEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTENLYF
Q
>6FG1:D|LIGHT CHAIN OF FAB NAA32
EIVMTQSPATLSVSPGERATLSCRASQSVSSNLAWYQQKPGQAPRLLIYGASTRATGIPARFSGSGSGTEFTLTISSLQS
EDFAVYYCQQYNNWPPLTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNALQSGNS
QESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHEGLSSPVTKSFNRGEC
>6FG1:H|HEAVY CHAIN OF FAB NATALIZUMAB
QVQLVQSGAEVKKPGASVKVSCKASGFNIKDTYIHWVRQAPGQRLEWMGRIDPANGYTKYDPKFQGRVTITADTSASTAY
MELSSLRSEDEAVYYCAREGYYGNYGVYAMDYWGQGTLVTVSSASTKGPSVFPLAPCSRSTSESTAALGCLVKDYFPEPV
TVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTKTYTCNVDHKPSNTKVDKRVESKYGPPENLYFQ
>6FG1:L|LIGHT CHAIN OF FAB NATALIZUMAB
DIQMTQSPSSLSASVGDRVTITCKTSQDINKYMAWYQQTPGKAPRLLIHYTSALQPGIPSRFSGSGSGRDYTFTISSLQP
EDIATYYCLQYDNLWTFGQGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNALQSGNSQE
SVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLSSPVTKSFNRGEC |
Identifiers
| PDB | 4IRZ, 6FG1, 6FG2 |
| CAS-ID | 189261-10-7 |
| RxCUI | 354770 |
| ChEMBL ID | CHEMBL1201607 |
| ChEBI ID | — |
| PubChem CID | — |
| DrugBank | DB00108 |
| UNII ID | 3JB47N2Q2P (ChemIDplus, GSRS) |
Target
Agency Approved
ITGA4
ITGA4
Organism
Homo sapiens
Gene name
ITGA4
Gene synonyms
CD49D
NCBI Gene ID
Protein name
integrin alpha-4
Protein synonyms
269C wild type, alpha 4 subunit of VLA-4 receptor, antigen CD49D, alpha-4 subunit of VLA-4 receptor, CD49 antigen-like family member D, CD49d, Integrin alpha-IV, very late activation protein 4 receptor, alpha 4 subunit, VLA-4 subunit alpha
Uniprot ID
Mouse ortholog
Itga4 (16401)
integrin alpha-4 (Q00651)
Alternate
No data
Variants
Clinical Variant
No data
Financial
Tysabri - Biogen
$
€
£
₣

Mock data
Subscribe for the real data
Subscribe for the real data

Mock data
Subscribe for the real data
Subscribe for the real data
Tysabri - Elan Corporation
$
€
£
₣

Mock data
Subscribe for the real data
Subscribe for the real data

Mock data
Subscribe for the real data
Subscribe for the real data
Tabular view
Trends
PubMed Central
Top Terms for Disease or Syndrome:

Mock data
Subscribe for the real data
Subscribe for the real data
Additional graphs summarizing 9,541 documents
View more details
Safety
Black-box Warning
Black-box warning for: Tysabri
Adverse Events
Top Adverse Reactions

Mock data
Subscribe for the real data
Subscribe for the real data
29,126 adverse events reported
View more details
Premium feature
Learn more about premium features at pharmakb.com
Learn more
