Gazyva, Gazyvaro(obinutuzumab)
Gazyva, Gazyvaro (obinutuzumab) is an antibody pharmaceutical. Obinutuzumab was first approved as Gazyva on 2013-11-01. It is used to treat follicular lymphoma and lymphoid leukemia in the USA. It has been approved in Europe to treat b-cell chronic lymphocytic leukemia. The pharmaceutical is active against B-lymphocyte antigen CD20.
Download report
Favorite
Commercial
Trade Name
FDA
EMA
Gazyva
Drug Products
FDA
EMA
Reference product - 351(a)
Reference product - 351(a)
Interchangeable product - 351(k)
Interchangeable product - 351(k)
Biosimilar product - 351(k)
Biosimilar product - 351(k)
Obinutuzumab
Tradename | Proper name | Company | Number | Date | Products |
|---|---|---|---|---|---|
| Gazyva | obinutuzumab | Genentech | N-125486 RX | 2013-11-01 | 1 products |
Labels
FDA
EMA
Brand Name | Status | Last Update |
|---|---|---|
| gazyva | Biologic Licensing Application | 2021-01-25 |
Agency Specific
FDA
EMA
Expiration | Code | ||
|---|---|---|---|
obinutuzumab, Gazyva, Genentech, Inc. | |||
| 2024-11-16 | Orphan excl. | ||
Patent Expiration
No data
HCPCS
Code | Description |
|---|---|
| J9301 | Injection, obinutuzumab, 10 mg |
Clinical
Clinical Trials
223 clinical trials
View more details

Mock data
Subscribe for the real data
Subscribe for the real data
Indications Phases 3
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| B-cell chronic lymphocytic leukemia | D015451 | C91.1 | 21 | 41 | 20 | — | 3 | 79 | |
| Non-hodgkin lymphoma | D008228 | C85.9 | 22 | 16 | 2 | — | — | 33 | |
| Large b-cell lymphoma diffuse | D016403 | C83.3 | 7 | 10 | 2 | — | — | 16 | |
| Lupus nephritis | D008181 | EFO_0005761 | — | 2 | 2 | — | — | 4 | |
| Systemic lupus erythematosus | D008180 | EFO_0002690 | M32 | — | — | 2 | — | — | 2 |
| Membranous glomerulonephritis | D015433 | EFO_0004254 | N03.2 | — | 1 | 1 | — | — | 2 |
| Nephrotic syndrome | D009404 | EFO_0004255 | N04 | — | — | 1 | — | — | 1 |
Indications Phases 2
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Mantle-cell lymphoma | D020522 | C83.1 | 5 | 10 | — | — | — | 12 | |
| Lymphoma | D008223 | C85.9 | 11 | 6 | — | — | — | 11 | |
| B-cell lymphoma | D016393 | 6 | 3 | — | — | — | 9 | ||
| Waldenstrom macroglobulinemia | D008258 | C88.0 | 2 | 4 | — | — | — | 5 | |
| Reactive arthritis | D016918 | EFO_0007460 | M02.3 | 2 | 4 | — | — | — | 5 |
| Burkitt lymphoma | D002051 | C83.7 | 3 | 2 | — | — | — | 4 | |
| Colorectal neoplasms | D015179 | 3 | 1 | — | — | — | 3 | ||
| B-cell lymphoma marginal zone | D018442 | C88.4 | 1 | 2 | — | — | — | 3 | |
| Hairy cell leukemia | D007943 | C91.4 | 2 | 2 | — | — | — | 3 | |
| Leukemia | D007938 | C95 | 1 | 2 | — | — | — | 3 |
Show 15 more
Indications Phases 1
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Breast neoplasms | D001943 | EFO_0003869 | C50 | 2 | — | — | — | — | 2 |
| Neoplasms | D009369 | C80 | 2 | — | — | — | — | 2 | |
| Hodgkin disease | D006689 | C81 | 1 | — | — | — | — | 1 | |
| Mycosis fungoides | D009182 | C84.0 | 1 | — | — | — | — | 1 | |
| Prostatic neoplasms | D011471 | C61 | 1 | — | — | — | — | 1 | |
| Renal cell carcinoma | D002292 | 1 | — | — | — | — | 1 | ||
| Ovarian neoplasms | D010051 | EFO_0003893 | C56 | 1 | — | — | — | — | 1 |
| Pancreatic neoplasms | D010190 | EFO_0003860 | C25 | 1 | — | — | — | — | 1 |
| Triple negative breast neoplasms | D064726 | 1 | — | — | — | — | 1 | ||
| Endometrial neoplasms | D016889 | EFO_0004230 | 1 | — | — | — | — | 1 |
Show 11 more
Indications Without Phase
No data
Epidemiology
Epidemiological information for investigational and approved indications
View more details
Drug
General
| Drug common name | OBINUTUZUMAB |
| INN | obinutuzumab |
| Description | Obinutuzumab (humanized mab) |
| Classification | Antibody |
| Drug class | monoclonal antibodies |
| Image (chem structure or protein) | 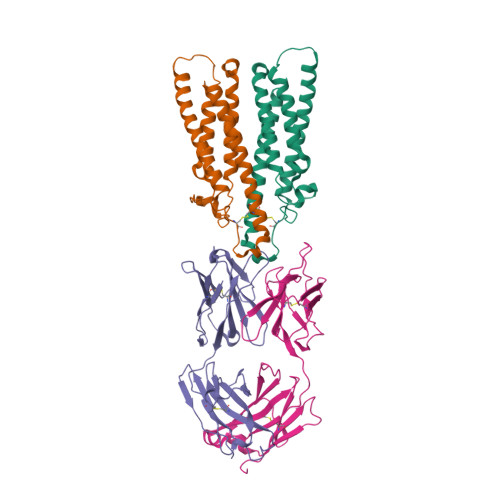 |
| Structure (InChI/SMILES or Protein Sequence) | >6Y9A:H|Obinutuzumab Fab heavy chain
VQLVQSGAEVKKPGSSVKVSCKASGYAFSYSWINWVRQAPGQGLEWMGRIFPGDGDTDYNGKFKGRVTITADKSTSTAYM
ELSSLRSEDTAVYYCARNVFDGYWLVYWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVSWN
SGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPK
>6Y9A:L|Obinutuzumab Fab Light chain
DIVMTQTPLSLPVTPGEPASISCRSSKSLLHSNGITYLYWYLQKPGQSPQLLIYQMSNLVSGVPDRFSGSGSGTDFTLKI
SRVEAEDVGVYYCAQNLELPYTFGGGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNALQ
SGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLSSPVTKSFNRGEC |
Identifiers
| PDB | 6Y97, 6Y9A |
| CAS-ID | 949142-50-1 |
| RxCUI | 974779 |
| ChEMBL ID | CHEMBL1743048 |
| ChEBI ID | — |
| PubChem CID | — |
| DrugBank | DB08935 |
| UNII ID | O43472U9X8 (ChemIDplus, GSRS) |
Target
Agency Approved
MS4A1
MS4A1
Organism
Homo sapiens
Gene name
MS4A1
Gene synonyms
CD20
NCBI Gene ID
Protein name
B-lymphocyte antigen CD20
Protein synonyms
B-lymphocyte cell-surface antigen B1, B-lymphocyte surface antigen B1, Bp35, CD20, CD20 antigen, CD20 receptor, Leukocyte surface antigen Leu-16, Membrane-spanning 4-domains subfamily A member 1, membrane-spanning 4-domains, subfamily A, member 1
Uniprot ID
Mouse ortholog
Ms4a1 (12482)
B-lymphocyte antigen CD20 (P19437)
Alternate
No data
Variants
Clinical Variant
No data
Financial
No data
Trends
PubMed Central
Top Terms for Disease or Syndrome:

Mock data
Subscribe for the real data
Subscribe for the real data
Additional graphs summarizing 3,735 documents
View more details
Safety
Black-box Warning
Black-box warning for: Gazyva
Adverse Events
Top Adverse Reactions
0 adverse events reported
View more details
Premium feature
Learn more about premium features at pharmakb.com
Learn more
