Onabotulinumtoxina
Botox (onabotulinumtoxina) is an unknown pharmaceutical. Onabotulinumtoxina was first approved as Botox cosmetic on 1991-12-09. It is used to treat blepharospasm, dystonia, fissure in ano, genetic skin diseases, and hyperhidrosis amongst others in the USA.
Download report
Favorite
Commercial
Therapeutic Areas
Therapeutic Area | MeSH |
|---|---|
| musculoskeletal diseases | D009140 |
| digestive system diseases | D004066 |
| nervous system diseases | D009422 |
| eye diseases | D005128 |
| urogenital diseases | D000091642 |
| hereditary congenital and neonatal diseases and abnormalities | D009358 |
| skin and connective tissue diseases | D017437 |
| signs and symptoms pathological conditions | D013568 |
Trade Name
FDA
EMA
Botox, Botox cosmetic
Drug Products
FDA
EMA
Reference product - 351(a)
Reference product - 351(a)
Interchangeable product - 351(k)
Interchangeable product - 351(k)
Biosimilar product - 351(k)
Biosimilar product - 351(k)
Labels
FDA
EMA
Brand Name | Status | Last Update |
|---|---|---|
| botox | Biologic Licensing Application | 2022-08-01 |
Indications
FDA
EMA
Indication | Ontology | MeSH | ICD-10 |
|---|---|---|---|
| blepharospasm | — | D001764 | G24.5 |
| dystonia | HP_0001332 | D004421 | G24 |
| fissure in ano | HP_0012390 | D005401 | K60.2 |
| genetic skin diseases | — | D012873 | — |
| hyperhidrosis | HP_0000975 | D006945 | — |
| migraine disorders | EFO_0003821 | D008881 | G43 |
| muscle rigidity | HP_0002063 | D009127 | — |
| overactive urinary bladder | EFO_1000781 | D053201 | N32.81 |
| strabismus | HP_0000486 | D013285 | H50.2 |
Agency Specific
FDA
EMA
Expiration | Code | ||
|---|---|---|---|
onabotulinumtoxina, Botox , Allergan, Inc. | |||
| 2026-06-20 | Orphan excl. | ||
onabotulinumtoxina, Botox Cosmetic, Allergan, Inc. | |||
| 2026-06-20 | Orphan excl. | ||
Patent Expiration
No data
ATC Codes
No data
HCPCS
Code | Description |
|---|---|
| J0585 | Injection, onabotulinumtoxina, 1 unit |
Clinical
Clinical Trials
17 clinical trials
View more details

Mock data
Subscribe for the real data
Subscribe for the real data
Indications Phases 4
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Cystic fibrosis | D003550 | EFO_0000390 | E84 | — | — | — | 1 | — | 1 |
Indications Phases 2
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Pyelonephritis | D011704 | EFO_1001141 | N10-N16 | — | 1 | — | — | — | 1 |
| Urinary tract infections | D014552 | EFO_0003103 | N39.0 | — | 1 | — | — | — | 1 |
| Cystitis | D003556 | EFO_1000025 | N30 | — | 1 | — | — | — | 1 |
| Bacterial skin diseases | D017192 | — | 1 | — | — | — | 1 | ||
| Lung diseases | D008171 | EFO_0003818 | J98.4 | — | 1 | — | — | — | 1 |
| Nontuberculous mycobacterium infections | D009165 | EFO_0007461 | A31.9 | — | 1 | — | — | — | 1 |
Indications Phases 1
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Wound infection | D014946 | 1 | — | — | — | — | 1 | ||
| Healthy volunteers/patients | — | 1 | — | — | — | — | 1 |
Indications Without Phase
No data
Epidemiology
Epidemiological information for investigational and approved indications
View more details
Drug
General
| Drug common name | ONABOTULINUMTOXINA |
| INN | — |
| Description | Botulinum neurotoxin type A precursor (BoNT/A) (Bontoxilysin A) |
| Classification | Neurotoxin |
| Drug class | cannabinol derivatives |
| Image (chem structure or protein) | 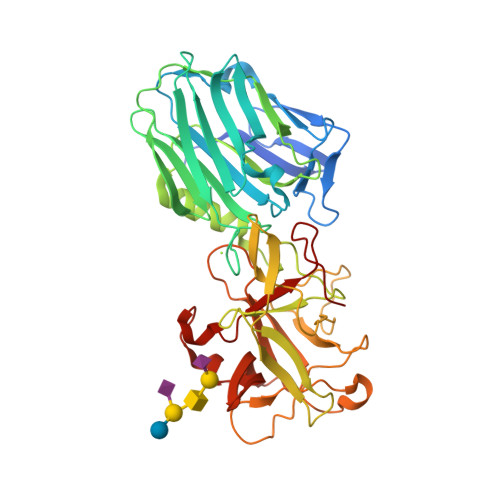 |
| Structure (InChI/SMILES or Protein Sequence) | >2VU9:A|BOTULINUM NEUROTOXIN A HEAVY CHAIN
MGSSHHHHHHSSGLVPRGSHMDTSILNLRYESNHLIDLSRYASKINIGSKVNFDPIDKNQIQLFNLESSKIEVILKNAIV
YNSMYENFSTSFWIRIPKYFNSISLNNEYTIINCMENNSGWKVSLNYGEIIWTLQDTQEIKQRVVFKYSQMINISDYINR
WIFVTITNNRLNNSKIYINGRLIDQKPISNLGNIHASNNIMFKLDGCRDTHRYIWIKYFNLFDKELNEKEIKDLYDNQSN
SGILKDFWGDYLQYDKPYYMLNLYDPNKYVDVNNVGIRGYMYLKGPRGSVMTTNIYLNSSLYRGTKFIIKKYASGNKDNI
VRNNDRVYINVVVKNKEYRLATNASQAGVEKILSALEIPDVGNLSQVVVMKSKNDQGITNKCKMNLQDNNGNDIGFIGFH
QFNNIAKLVASNWYNRQIERSSRTLGCSWEFIPVDDGWGERPLQ |
Identifiers
| PDB | 2G7P, 2VU9, 2VUA, 3BTA, 3DDA, 3DDB, 3DS9, 3DSE, 3FUO, 3K3Q, 3QIX, 3QIY, 3QIZ, 3QJ0, 3QW5, 3QW6, 3QW7, 3QW8, 3ZUR, 3ZUS, 4EJ5, 4EL4, 4ELC, 4HEV, 4IQP, 4JRA, 4KS6, 4KTX, 4KUF, 4ZJX, 5JLV, 5JMC, 5L21, 5MK6, 5MK7, 5MOY, 5TPB, 5TPC, 5V8P, 5V8R, 5V8U, 5VGV, 5VGX, 6DKK, 6ES1, 6MHJ, 6XCB, 6XCC, 6XCD, 6XCE, 6XCF, 7N18 |
| CAS-ID | — |
| RxCUI | — |
| ChEMBL ID | CHEMBL1201574 |
| ChEBI ID | — |
| PubChem CID | — |
| DrugBank | DB00083 |
| UNII ID | — |
Target
Agency Approved
No data
Alternate
No data
Variants
Clinical Variant
No data
Financial
Botox - Allergan
$
€
£
₣

Mock data
Subscribe for the real data
Subscribe for the real data

Mock data
Subscribe for the real data
Subscribe for the real data
Botox - Warner Chilcott
$
€
£
₣

Mock data
Subscribe for the real data
Subscribe for the real data

Mock data
Subscribe for the real data
Subscribe for the real data
Tabular view
Trends
PubMed Central
Top Terms for Disease or Syndrome:

Mock data
Subscribe for the real data
Subscribe for the real data
Additional graphs summarizing 6,954 documents
View more details
Safety
Black-box Warning
Black-box warning for: Botox
Adverse Events
Top Adverse Reactions

Mock data
Subscribe for the real data
Subscribe for the real data
226 adverse events reported
View more details
Premium feature
Learn more about premium features at pharmakb.com
Learn more
