Utomilumab
Utomilumab is an antibody pharmaceutical. It is currently being investigated in clinical studies. The pharmaceutical is active against tumor necrosis factor receptor superfamily member 9.
Download report
Favorite
Commercial
Therapeutic Areas
No data
Trade Name
FDA
EMA
No data
Drug Products
FDA
EMA
New Drug Application (NDA)
New Drug Application (NDA)
Abbreviated New Drug Application (ANDA)
Abbreviated New Drug Application (ANDA)
No data
Labels
FDA
EMA
No data
Indications
FDA
EMA
No data
Agency Specific
FDA
EMA
No data
Patent Expiration
No data
ATC Codes
No data
HCPCS
No data
Clinical
Clinical Trials
17 clinical trials
View more details

Mock data
Subscribe for the real data
Subscribe for the real data
Indications Phases 4
No data
Indications Phases 2
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Breast neoplasms | D001943 | EFO_0003869 | C50 | 1 | 2 | — | — | — | 3 |
| Triple negative breast neoplasms | D064726 | — | 1 | — | — | — | 1 | ||
| Invasive hydatidiform mole | D002820 | D39.2 | — | 1 | — | — | — | 1 | |
| Prostatic neoplasms | D011471 | C61 | 1 | 1 | — | — | — | 1 | |
| Castration-resistant prostatic neoplasms | D064129 | 1 | 1 | — | — | — | 1 | ||
| Oropharyngeal neoplasms | D009959 | — | 1 | — | — | — | 1 |
Indications Phases 1
Indication | MeSH | Ontology | ICD-10 | Ph 1 | Ph 2 | Ph 3 | Ph 4 | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Follicular lymphoma | D008224 | C82 | 2 | — | — | — | — | 2 | |
| Mantle-cell lymphoma | D020522 | C83.1 | 1 | — | — | — | — | 1 | |
| Colorectal neoplasms | D015179 | 1 | — | — | — | — | 1 | ||
| Renal cell carcinoma | D002292 | 1 | — | — | — | — | 1 | ||
| Non-hodgkin lymphoma | D008228 | C85.9 | 1 | — | — | — | — | 1 | |
| Squamous cell carcinoma of head and neck | D000077195 | 1 | — | — | — | — | 1 | ||
| Melanoma | D008545 | 1 | — | — | — | — | 1 |
Indications Without Phase
No data
Epidemiology
Epidemiological information for investigational and approved indications
View more details
Drug
General
| Drug common name | UTOMILUMAB |
| INN | utomilumab |
| Description | CD137, a member of the tumor necrosis factor (TNF) receptor family, is a type 1 transmembrane protein, expressed on surfaces of leukocytes and non-immune cells. Its alternative names are tumor necrosis factor receptor superfamily member 9 (TNFRSF9), 4-1BB, and induced by lymphocyte activation (ILA). It is of interest to immunologists as a co-stimulatory immune checkpoint molecule, and as a potential target in cancer immunotherapy. |
| Classification | Antibody |
| Drug class | monoclonal antibodies |
| Image (chem structure or protein) | 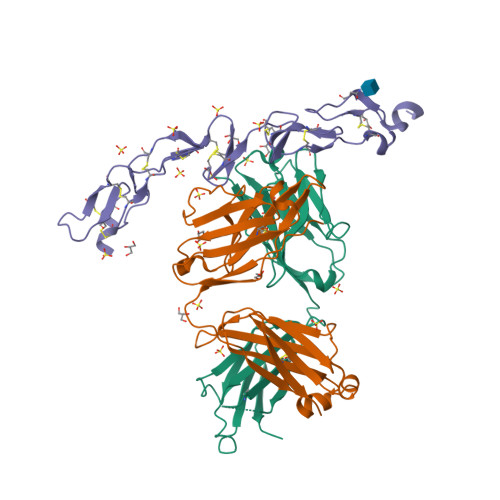 |
| Structure (InChI/SMILES or Protein Sequence) | >6MI2:A,D|Utomilumab Fab heavy chain
EVQLVQSGAEVKKPGESLRISCKGSGYSFSTYWISWVRQMPGKGLEWMGKIYPGDSYTNYSPSFQGQVTISADKSISTAY
LQWSSLKASDTAMYYCARGYGIFDYWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVSWNSG
ALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCAAAHHHHHHHH
>6MI2:B,E|Utomilumab Fab lambda chain
SYELTQPPSVSVSPGQTASITCSGDNIGDQYAHWYQQKPGQSPVLVIYQDKNRPSGIPERFSGSNSGNTATLTISGTQAM
DEADYYCATYTGFGSLAVFGGGTKLTVLGQPKAAPSVTLFPPSSEELQANKATLVCLISDFYPGAVTVAWKADSSPVKAG
VETTTPSKQSNNKYAASSYLSLTPEQWKSHRSYSCQVTHEGSTVEKTVAPTECS
>6MI2:F,C|Tumor necrosis factor receptor superfamily member 9
QDPCSNCPAGTFCDNNRNQICSPCPPNSFSSAGGQRTCDICRQCKGVFRTRKECSSTSNAECDCTPGFHCLGAGCSMCEQ
DCKQGQELTKKGCKDCCFGTFNDQKRGICRPWTNCSLDGKSVLVNGTKERDVVCGPSPENLYFQG |
Identifiers
| PDB | 6A3W, 6MI2 |
| CAS-ID | — |
| RxCUI | — |
| ChEMBL ID | CHEMBL3707324 |
| ChEBI ID | — |
| PubChem CID | — |
| DrugBank | — |
| UNII ID | 6YY8O697VF (ChemIDplus, GSRS) |
Target
Agency Approved
TNFRSF9
TNFRSF9
Organism
Homo sapiens
Gene name
TNFRSF9
Gene synonyms
CD137, ILA
NCBI Gene ID
Protein name
tumor necrosis factor receptor superfamily member 9
Protein synonyms
4-1BB ligand receptor, CD137, CD137 antigen, CDw137, homolog of mouse 4-1BB, induced by lymphocyte activation (ILA), interleukin-activated receptor, homolog of mouse Ly63, receptor protein 4-1BB, T cell antigen ILA, T-cell antigen 4-1BB homolog, T-cell antigen ILA
Uniprot ID
Mouse ortholog
Tnfrsf9 (21942)
tumor necrosis factor receptor superfamily member 9 (P20334)
Alternate
No data
Variants
Clinical Variant
No data
Financial
No data
Trends
PubMed Central
Top Terms for Disease or Syndrome:

Mock data
Subscribe for the real data
Subscribe for the real data
Additional graphs summarizing 413 documents
View more details
Safety
Black-box Warning
No Black-box warning
Adverse Events
Top Adverse Reactions

Mock data
Subscribe for the real data
Subscribe for the real data
21 adverse events reported
View more details
Premium feature
Learn more about premium features at pharmakb.com
Learn more
